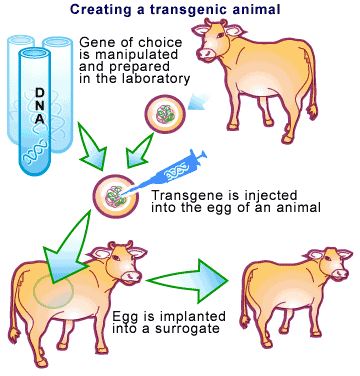
-
Established in 1986 in the United States, Pharming was created to insert genes that code for useful pharmaceuticals into animals or plants that would not naturally express those genes. But as a consequence, the animal makes more of that gene, not being able to regulate it, so that it can be purified and then used as a drug. These can be delivered by directly eating the plant, or drinking the milk. The products of pharming, recombinant proteins, have potentially greater efficiency and fewer side effects than regular drugs, because there action can be easily targeted toward the problem, making pharming better than traditional ways of treating illness. Companies in this industry hope that someday pharming might be used to cure some of the more severe diseases such as cancer, diabetes, HIV, etc. (David Gillespie, pharming for farmaceuticals)
- Recombinant proteins, the products of pharming, are most commonly produced using bacteria or yeast in bioreactor, but pharming offers the advantage to the producer that it does not require expensive infrastructure, and production capacity can be quickly scaled to meet demand. It is estimated that the expense of producing a recombinant protein drug via pharming will be less than 70% of the current cost. In the United States, Transgenic plants including but not limited to those that produce pharmaceuticals, are regulated by three government agencies, which comprise the Coordinated Framework for Regulation of Biotechnology established in 1986: United States Department of Agriculture Animal and Plant Health Inspection Service, United States Environmental Protection Agency, and the United States Department of Health and Human Services Food and Drug Administration. (David Gillespie, pharming for farmaceuticals)
|